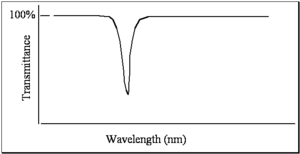Transition Dipole Moment
Return to Absorption and Emission Menu Next Topic
Spectroscopy
When measuring a spectrum, the simplest thing you can do is have a light source where you can vary the wavelength. The intensity of the light can be measured with a detector as a function of wavelength or as a function of frequency. Afterwards, by placing a sample in the detector called a spectrometer, you can take the ratio. In old spectrometers, and even still today in the new spectrometers, there are literally two paths: one for reference beam and one for sample beam. The spectrum that is referred to as a transmittance spectrum can be obtained by finding the ratio of the light that is going through the sample, dividing by the light that has been unperturbed, and multiplying that by a hundred. If the molecule does not absorb any light, then all the light goes through and there is a 100% transmittance. If the molecule absorbs 99% of the light, then there is a 1% transmittance. This is one way of doing that and that is referred to as transmittance spectrum.
For reasons of unknown origins, people look at different spectra in different modes. Typically when looking at color, chemists, not physicists, look at spectra in a mode that is referred to as absorbance. But when looking at infrared spectra we typically consider them in terms of transmittance. Absorbance and transmittance are related to one another by the Beer- Lambert law.
<math>log \frac {l_0}{l} = \varepsilon cl = A\,\!</math>
Where A is the absorbance (a unitless quantity),
l is the path length of the sample in cm,
c is the concentration of the chromophore in the medium in mol-l-1
ϵ is the molar absorptivity or extinction coefficient with units of l-mol-1cm-1.
The extinction coefficient characterizes the ability of a molecule to absorb light at a given wavelength
What this law simply says is that the log base 10 of the transmittance is equal to εcl or the absorbance. So the absorbance is simply the log of I0 over I. Keep in mind that absorbance is a unitless quantity. It turns out that absorbance can be related back to some characteristic features of the molecule and that is one of the reasons why it is used today. In the formula εcl, c is the concentration of the molecule and l is the path length. This formula shows that if there is a certain amount of a molecule and the path length is doubled, twice as much light will be absorbed. At least with the first order, if the concentration of a molecule is doubled, assuming that there are no intermolecular effects, twice as much light will be absorbed. Those are not molecular characteristics; they are characteristics of your sample. ε is related to how well the molecule absorbs light and it is referred to as the molar absorbtivity or the extinction coefficient. Since A (absorbance) is unitless, c is concentration given in moles per liter, and path length is typically given in cm, the units of the extinction coefficient are in: Liter mol-1 cm-1 liter or inverse molar, inverse centimeters. The extinction coefficient characterizes the ability of the molecule to absorb light of the given wavelength. It doesn’t matter whether the sample is liquid or gas. Chemist use log base 10 for the liquid phase, in gas phase spectroscopy you use an absorption cross section with a natural log. Often electrical engineers use natural logs as well.