Difference between revisions of "Sigma and pi Orbitals"
Jump to navigation
Jump to search
Cmditradmin (talk | contribs) |
Cmditradmin (talk | contribs) |
||
| Line 4: | Line 4: | ||
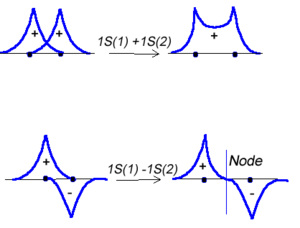
Molecular orbital theory was developed in the early part of the last century to help rationalize why bonds form and to explaint the properties of molecules. In molecular orbital theory the atomic orbitals from each atom can overlap with those on other atoms. Since the atomic orbitals are wavefunctions and behave like waves it is possible for them to overlap in a constructive manner to form a bonding orbital. | Molecular orbital theory was developed in the early part of the last century to help rationalize why bonds form and to explaint the properties of molecules. In molecular orbital theory the atomic orbitals from each atom can overlap with those on other atoms. Since the atomic orbitals are wavefunctions and behave like waves it is possible for them to overlap in a constructive manner to form a bonding orbital. | ||
<math>\Psi_{new} = \Psi_1 + \Psi_2\,\!</math> | <math>\Psi_{new} = \Psi_1 + \Psi_2\,\!</math> | ||
Or the two functions can combine in a destructive manner. | |||
<math>\Psi_{new} = \Psi_1 - \Psi_2\,\!</math> | |||
[[Image:2sigma_orbitals.png|thumb|300px|]] | |||
Revision as of 10:21, 19 May 2009
Return to Molecular Orbitals Menu | Next Topic
Molecular orbital theory was developed in the early part of the last century to help rationalize why bonds form and to explaint the properties of molecules. In molecular orbital theory the atomic orbitals from each atom can overlap with those on other atoms. Since the atomic orbitals are wavefunctions and behave like waves it is possible for them to overlap in a constructive manner to form a bonding orbital.
<math>\Psi_{new} = \Psi_1 + \Psi_2\,\!</math>
Or the two functions can combine in a destructive manner.
<math>\Psi_{new} = \Psi_1 - \Psi_2\,\!</math>