Difference between revisions of "Passive Optical Polymers"
Cmditradmin (talk | contribs) |
Cmditradmin (talk | contribs) m |
||
| (21 intermediate revisions by 2 users not shown) | |||
| Line 7: | Line 7: | ||
</tr> | </tr> | ||
</table> | </table> | ||
Passive devices are polymers that are non-electro optical materials. These polymers just transmit the light but they can be used for waveguides and filters for optical networks. | |||
== Passive Optical Polymers for Integrated Optical Devices | == Passive Optical Polymers for Integrated Optical Devices -Properties and Requirements == | ||
The challenge with passive optical materials is match their physical characteristics with the requirements in applied devices. | |||
=== Passive waveguide materials requirements === | === Passive waveguide materials requirements === | ||
What is the perfect passive waveguide material? | What is the perfect passive waveguide material? | ||
*Low SM waveguide loss: 0.1 dB/cm. This 10K | *Low SM waveguide loss: 0.1 dB/cm. This this can be 10K less than the requirement for fiber because it operates over a very small distance. | ||
*Low birefringence: 0.0002 – Lasers are well polarized but after a few meters of fiber the polarization becomes randomized. The material must not depend on polarization. | *Low birefringence: 0.0002 – Lasers are well polarized but after a few meters of fiber the polarization becomes randomized. The material must not depend on polarization. | ||
| Line 53: | Line 31: | ||
*Good moisture and chemical resistance | *Good moisture and chemical resistance | ||
=== Polymer Optical Properties=== | |||
== | *Refractive index n = 1.3-1.6 | ||
*dn/dT ~ 10<sup>-4</sup> - 10<sup>-3</sup> /°C | |||
*Birefringence = 0 - 0.1 (small) | |||
*Index tunability for optimal match to fiber. An 8 micron core must be perfectly matched or else there is extreme loss. | |||
*UV optical properties (for photocured systems) | |||
Polymethacrylate (PMMA, also known as lucite) is a transparent polymer that has been used for waveguides. It is also used in glazing. Its’ Tg is about 100 deg C, which is a little low for some applications. | |||
[[Image:Teflon_af.png|thumb| | === Polymer Thermomechanical Properties=== | ||
*Glass transition(-40°C – 300°C)- it is good to get as high as possible before decomposition temperature. | |||
*Susceptibility to solvents- | |||
*Rheology/viscosity- | |||
*Hard/rubbery( xlink density) | |||
*Coefficient of thermal expansion (CTE) ~ 10-4 /oC | |||
*Phase transitions near operating range- a disadvantage because they may may melt and become disordered. | |||
== Types of Optical polymers == | |||
[[Image:Pmma.png|thumb|100px|Poly(methylmethacrylate)]] | |||
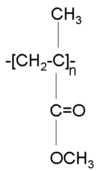
Polymethacrylate (PMMA, also known as lucite) is a transparent polymer that has been used for waveguides. It is also used in glazing. Its’ Tg is about 100 ° C, which is a little low for some applications. | |||
[[Image:Teflon_af.png|thumb|100px|Teflon AF]] | |||
Teflon AF is fully fluorinated. This helps eliminate hydrogen vibrational absorption in the 1000-2000 nm range. It also has a refractive index less than water, (n= 1.29) They are high molecular weight polymers (mw=10 5 to 10 6) | Teflon AF is fully fluorinated. This helps eliminate hydrogen vibrational absorption in the 1000-2000 nm range. It also has a refractive index less than water, (n= 1.29) They are high molecular weight polymers (mw=10 5 to 10 6) | ||
[[Image:Halogenated_uv_acrylates.png|thumb|300px|Halogenated | [[Image:Halogenated_uv_acrylates.png|thumb|300px|Halogenated UV acrylates]] | ||
UV acrylates]] | |||
Halogenated UV acrylates are very light | Halogenated UV acrylates are very light as monomers and don’t become polymers until the device is made. | ||
=== Organically-modified sol-gels === | === Organically-modified sol-gels === | ||
| Line 70: | Line 69: | ||
Sol gels are related to the monomeric materials. They have organic and inorganic pieces to them. | Sol gels are related to the monomeric materials. They have organic and inorganic pieces to them. | ||
There is a chemical route for production of glass-like material from metallo-organic precursors. They can be low temperature processed but are more reliable and stable. They can be tuned by mixing | There is a chemical route for production of glass-like material from metallo-organic precursors. They can be low-temperature processed but are more reliable and stable. They can be tuned by mixing and matching monomers. They can be photo-patterned. | ||
Advantages: | Advantages: | ||
Low temperature processing (< | *Low temperature processing (<250°C) | ||
Thick, multilayer film deposition | *Thick, multilayer film deposition | ||
Refractive index tuning (doping) | *Refractive index tuning (doping) | ||
High stability (inorganic network) | *High stability (due to inorganic network) | ||
Can be used to make low loss waveguides | *Can be used to make low loss waveguides | ||
Photo-patternable (due to the organic element) | *Photo-patternable (due to the organic element) | ||
Low cost (spin coating and UV patterning) | *Low cost manufacturing (spin coating and UV patterning) | ||
Compatible with heterogeneous integration | *Compatible with heterogeneous integration | ||
Potential EO active doping | *Potential EO active doping | ||
see Enami 2003 <ref>Enami, et. al. APL 83, 4692 (2003)</ref> | |||
see Zhang 2005 <ref>Zhang, et. al. OL 30, 117 (2005)</ref> | |||
=== Sol-gel waveguide materials === | === Sol-gel waveguide materials === | ||
[[Image:Sol_gel.png|thumb|300px|]] | [[Image:Sol_gel.png|thumb|300px|A sol-gel has an organic network (left) and an inorganic glass-like network (right) ]] | ||
A two component sol gel with methacryloyloxy propyltrimethoxysilane and zirconium(IV)-n-propoxide. The top component has an acrylate group that | A two-component sol-gel with methacryloyloxy propyltrimethoxysilane and zirconium(IV)-n-propoxide. The top component has an acrylate group that forms an organic network by photopolymerization. | ||
On the | On the (right) side is an inorganic or glass network built around silicon bonded to methoxy groups. In the sol-gel reaction these become OH groups, and Si-O bonds form essentially making glass. The zirconium propoxide is used to adjust the refractive index. By changing the fraction of the organic and inorganic components the refractive index can be adjusted | ||
In waveguides A 95/5 molar ratio of methacryloyloxy propyltrimethoxysilane to zirconium(IV)-n-propoxide is used as a cladding; 85/15 is used for the sol-gel core | In waveguides A 95/5 molar ratio of methacryloyloxy propyltrimethoxysilane to zirconium(IV)-n-propoxide is used as a cladding; 85/15 is used for the sol-gel core over a useful range. The solution is hydrolyzed with 0.1 N HCl. | ||
Polymers waveguides push below 0.1 dB/cm | Polymers waveguides can push loss below 0.1 dB/cm | ||
Over the last 25 years loss has | Over the last 25 years loss has improved greatly. This rate of improvement depends on the accumulated knowledge of many workers in the field. | ||
The state of the art waveguide polymer now has loss below .1 dB/cm. We are now competitive with silica on silicon technology but at a much lower cost and is low temperature processable. | |||
=== Absorption loss === | === Absorption loss === | ||
| Line 109: | Line 111: | ||
A fundamental vibration such as carbon – hydrogen is not a problem however the higher harmonics of this vibrations leads to absorption. | A fundamental vibration such as carbon – hydrogen is not a problem however the higher harmonics of this vibrations leads to absorption. | ||
Molecular vibrations essentially are high n states of an anharmonic oscillator. This is the dominant source of absorption loss in the near IR . | |||
:<math>E_0 \Rightarrow \sqrt \frac {k} {mu}\,\!</math> | :<math>E_0 \Rightarrow \sqrt \frac {k} {mu}\,\!</math> | ||
| Line 115: | Line 118: | ||
:<math>\mu = \frac {m_c m_x} {m_c + m_x}\,\!</math> | :<math>\mu = \frac {m_c m_x} {m_c + m_x}\,\!</math> | ||
The energy of vibration of a spring like systems is proportional to the square root of the spring constant divided by mu the effective mass of the spring. If | The energy of vibration of a spring like systems is proportional to the square root of the spring constant divided by μ the effective mass of the spring. If m<sub>x</sub> is hydrogen and m<sub>c</sub> is carbon then the carbons cancel out and the hydrogen becomes the dominant player. | ||
Using halogen to substitute for hydrogen makes mu larger and therefore makes | Using halogen to substitute for hydrogen makes μ larger and therefore makes E<sub>0</sub> smaller. | ||
Recall Beer’s law: | Recall Beer’s law: | ||
| Line 129: | Line 132: | ||
See Eldada 1998 <ref>L. Eldada, A. Nahata & J. Yardley, Proc SPIE 3288, 175 (1998)</ref> | See Eldada 1998 <ref>L. Eldada, A. Nahata & J. Yardley, Proc SPIE 3288, 175 (1998)</ref> | ||
Key Strategies | |||
*Most polymers absorb in near IR (1300 nm- 1600 nm) | *Most polymers absorb in near IR (1300 nm- 1600 nm) | ||
| Line 138: | Line 143: | ||
To measure absorption you must have a large quantity of the polymer sample. A very pure polished 4.5 cm plug is prepared, measured with a spectrophotometer and absorbance is expressed as an absorption coefficient. This high quality sample of PMMA shows two vibrational peaks. | To measure absorption you must have a large quantity of the polymer sample. A very pure polished 4.5 cm plug is prepared, measured with a spectrophotometer and absorbance is expressed as an absorption coefficient. This high quality sample of PMMA shows two vibrational peaks. | ||
To convert absorption coefficient ( | To convert absorption coefficient (α 1/cm) into decibals: | ||
Loss (dB/cm) = :<math>4.34 \alpha\,\!</math> | Loss (dB/cm) = :<math>4.34 \alpha\,\!</math> | ||
| Line 144: | Line 149: | ||
See Norwood 1991 <ref>Norwood, et. al. in Polymers for Lightwave and Integrated Optics, ed. L.A. Hornak (1991) | See Norwood 1991 <ref>Norwood, et. al. in Polymers for Lightwave and Integrated Optics, ed. L.A. Hornak (1991) | ||
ks.</ref> | ks.</ref> | ||
<br clear='all'> | |||
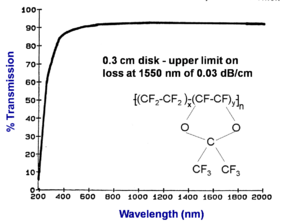
[[Image:Teflon_af_absorption.png|thumb|300px|]] | |||
Transmission spectrum of Teflon® AF | Transmission spectrum of Teflon® AF | ||
A thinner sample of fluorinated polymer (Teflon® AF) containing no hydrogen has very low loss across the entire spectrum from 800nm to 2000 nm. This establishes the upper limit on loss at 1550 nm of 0.03 dB/cm. In this case a .3 cm sample was used and this makes the measurement less reliable. This is 10-20 times less loss than PMMA value. | A thinner sample of fluorinated polymer (Teflon® AF) containing no hydrogen has very low loss across the entire spectrum from 800nm to 2000 nm. This establishes the upper limit on loss at 1550 nm of 0.03 dB/cm. In this case a .3 cm sample was used and this makes the measurement less reliable than if a thicker sample had been used. This is 10-20 times less loss than PMMA value. | ||
<br clear='all'> | |||
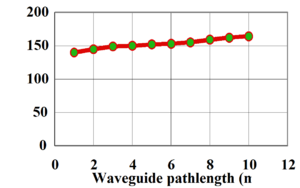
[[Image:Sol_gel_loss.png|thumb|300px|Slab loss of standard sol-gel at 1310nm | [[Image:Sol_gel_loss.png|thumb|300px|Slab loss of standard sol-gel at 1310nm]] | ||
]] | |||
This shows the intensity of light (transmission) passing through an optical waveguide containing a standard sol-gel (containing hydrogen) as a function of pathlength. This shows a loss of 0.8 dB/cm. | This shows the intensity of light (transmission) passing through an optical waveguide containing a standard sol-gel (containing hydrogen) as a function of pathlength. This shows a loss of 0.8 dB/cm. | ||
<br clear='all'> | |||
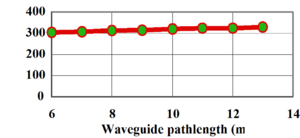
[[Image:Sol_ge_fluorinated_loss.png|thumb|300px|Slab loss of fluorinated sol-gel at 1310nm]] | |||
A partially fluorinated sol-gel shows higher transmission and a loss of 0.8 dB/cm. The greatest gains can be had by substituting all the hydrogen with halogens. | A partially fluorinated sol-gel shows higher transmission and a loss of 0.8 dB/cm. The greatest gains can be had by substituting all the hydrogen with halogens. | ||
<br clear='all'> | <br clear='all'> | ||
== References == | |||
<references/> | |||
[[category:photonics applications]] | |||
<table id="toc" style="width: 100%"> | <table id="toc" style="width: 100%"> | ||
Latest revision as of 13:25, 29 December 2009
| Previous Topic | Return to Organic Photonics Applications Menu | Next Topic |
Passive devices are polymers that are non-electro optical materials. These polymers just transmit the light but they can be used for waveguides and filters for optical networks.
Passive Optical Polymers for Integrated Optical Devices -Properties and Requirements
The challenge with passive optical materials is match their physical characteristics with the requirements in applied devices.
Passive waveguide materials requirements
What is the perfect passive waveguide material?
- Low SM waveguide loss: 0.1 dB/cm. This this can be 10K less than the requirement for fiber because it operates over a very small distance.
- Low birefringence: 0.0002 – Lasers are well polarized but after a few meters of fiber the polarization becomes randomized. The material must not depend on polarization.
- Core/cladding system with good control over the refractive index providing for mode match to optical fiber
- Compatible with lithographic processes – We want to take advantage of knowledge and practices of semiconductor industry – lithographic process are quite scalable. As the volume goes up the price goes down.
- Environmental stability: These materials may be stored in extreme temperatures even if they are used in a controlled environement (Telcordia 1209, 1221 standards)
- Optical power handling > 23 dBm - (0dBm =1 mw, -dBM <1 mw, 23 dBM = 100 mw)
- Good moisture and chemical resistance
Polymer Optical Properties
- Refractive index n = 1.3-1.6
- dn/dT ~ 10-4 - 10-3 /°C
- Birefringence = 0 - 0.1 (small)
- Index tunability for optimal match to fiber. An 8 micron core must be perfectly matched or else there is extreme loss.
- UV optical properties (for photocured systems)
Polymer Thermomechanical Properties
- Glass transition(-40°C – 300°C)- it is good to get as high as possible before decomposition temperature.
- Susceptibility to solvents-
- Rheology/viscosity-
- Hard/rubbery( xlink density)
- Coefficient of thermal expansion (CTE) ~ 10-4 /oC
- Phase transitions near operating range- a disadvantage because they may may melt and become disordered.
Types of Optical polymers
Polymethacrylate (PMMA, also known as lucite) is a transparent polymer that has been used for waveguides. It is also used in glazing. Its’ Tg is about 100 ° C, which is a little low for some applications.
Teflon AF is fully fluorinated. This helps eliminate hydrogen vibrational absorption in the 1000-2000 nm range. It also has a refractive index less than water, (n= 1.29) They are high molecular weight polymers (mw=10 5 to 10 6)
Halogenated UV acrylates are very light as monomers and don’t become polymers until the device is made.
Organically-modified sol-gels
Sol gels are related to the monomeric materials. They have organic and inorganic pieces to them.
There is a chemical route for production of glass-like material from metallo-organic precursors. They can be low-temperature processed but are more reliable and stable. They can be tuned by mixing and matching monomers. They can be photo-patterned.
Advantages:
- Low temperature processing (<250°C)
- Thick, multilayer film deposition
- Refractive index tuning (doping)
- High stability (due to inorganic network)
- Can be used to make low loss waveguides
- Photo-patternable (due to the organic element)
- Low cost manufacturing (spin coating and UV patterning)
- Compatible with heterogeneous integration
- Potential EO active doping
see Enami 2003 [1]
see Zhang 2005 [2]
Sol-gel waveguide materials
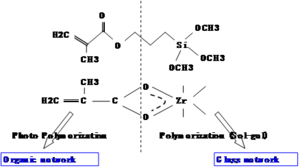
A two-component sol-gel with methacryloyloxy propyltrimethoxysilane and zirconium(IV)-n-propoxide. The top component has an acrylate group that forms an organic network by photopolymerization.
On the (right) side is an inorganic or glass network built around silicon bonded to methoxy groups. In the sol-gel reaction these become OH groups, and Si-O bonds form essentially making glass. The zirconium propoxide is used to adjust the refractive index. By changing the fraction of the organic and inorganic components the refractive index can be adjusted
In waveguides A 95/5 molar ratio of methacryloyloxy propyltrimethoxysilane to zirconium(IV)-n-propoxide is used as a cladding; 85/15 is used for the sol-gel core over a useful range. The solution is hydrolyzed with 0.1 N HCl.
Polymers waveguides can push loss below 0.1 dB/cm
Over the last 25 years loss has improved greatly. This rate of improvement depends on the accumulated knowledge of many workers in the field.
The state of the art waveguide polymer now has loss below .1 dB/cm. We are now competitive with silica on silicon technology but at a much lower cost and is low temperature processable.
Absorption loss
Electronic absorption is neglible for passive materials. In electro-optic materials this kind of loss is significant. Electronic absorption – given by Urbach tail is usually neglible in the near infrared for passive materials.
- <math>\alpha(\omega) = A exp[\sigma (\hbar \omega – \hbar \omega_0) / kT ]\,\!</math>
Vibrational absorption
A fundamental vibration such as carbon – hydrogen is not a problem however the higher harmonics of this vibrations leads to absorption.
Molecular vibrations essentially are high n states of an anharmonic oscillator. This is the dominant source of absorption loss in the near IR .
- <math>E_0 \Rightarrow \sqrt \frac {k} {mu}\,\!</math>
- <math>\mu = \frac {m_c m_x} {m_c + m_x}\,\!</math>
The energy of vibration of a spring like systems is proportional to the square root of the spring constant divided by μ the effective mass of the spring. If mx is hydrogen and mc is carbon then the carbons cancel out and the hydrogen becomes the dominant player.
Using halogen to substitute for hydrogen makes μ larger and therefore makes E0 smaller.
Recall Beer’s law:
- <math>P_{out}= P_{in}e^{-\alpha L}\,\!</math>
Reducing Absorption in polymers
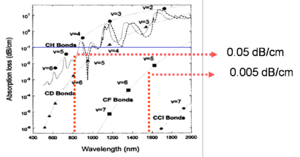
One strategy to improve the absorption is to substitute hydrogen for a heavier version of hydrogen in order to decrease the vibrational energy. The graph shows that by replacing hydrogen with deuterium (upper graph), which is double the mass, does improve the performance at 1310 but this works against you 1350nm. By replacing H with a halogen you can get losses down to .005 dB/cm
See Eldada 1998 [3]
Key Strategies
- Most polymers absorb in near IR (1300 nm- 1600 nm)
- C-H and OH groups have overtones
- Replace hydrogen with fluorine to reduce overtones
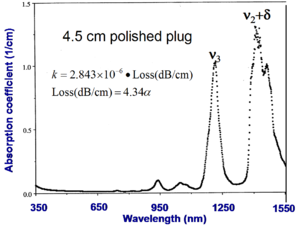
To measure absorption you must have a large quantity of the polymer sample. A very pure polished 4.5 cm plug is prepared, measured with a spectrophotometer and absorbance is expressed as an absorption coefficient. This high quality sample of PMMA shows two vibrational peaks.
To convert absorption coefficient (α 1/cm) into decibals:
Loss (dB/cm) = :<math>4.34 \alpha\,\!</math>
See Norwood 1991 [4]
Transmission spectrum of Teflon® AF
A thinner sample of fluorinated polymer (Teflon® AF) containing no hydrogen has very low loss across the entire spectrum from 800nm to 2000 nm. This establishes the upper limit on loss at 1550 nm of 0.03 dB/cm. In this case a .3 cm sample was used and this makes the measurement less reliable than if a thicker sample had been used. This is 10-20 times less loss than PMMA value.
This shows the intensity of light (transmission) passing through an optical waveguide containing a standard sol-gel (containing hydrogen) as a function of pathlength. This shows a loss of 0.8 dB/cm.
A partially fluorinated sol-gel shows higher transmission and a loss of 0.8 dB/cm. The greatest gains can be had by substituting all the hydrogen with halogens.
References
| Previous Topic | Return to Organic Photonics Applications Menu | Next Topic |