Electronic Coupling Between Orbitals
Return to Molecular Orbitals Menu | Next Topic
We will discuss the electronic structure of π-conjugated organic molecules at various levels of complexity. π-conjugated molecules have a sigma electron framework and π electron framework.
Here we start at the simplest level that is basically derived from a Hückel Molecular Orbital approach in order to understand conjugated systems.
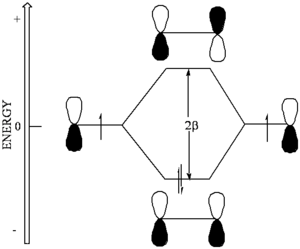
In this model, orbitals that are on atoms that are directly σ-bonded to one another and whose p-orbitals are in a plane can interact. This interaction is called the "electronic coupling" between the orbitals and has units of energy.
In the Hückel approximation this energy is the same for all carbon p-orbitals that are adjacent to on another and is called Β.
For atoms that are not adjacent to one another Β is taken to be zero. Thus in the Hückel approximation there is no interaction between nonadjacent atoms.
Through this coupling of atomic orbitals, molecular orbitals can formed by a constructive and destructive combination of the atomic orbitals.