Difference between revisions of "Electronic Coupling Between Orbitals"
Cmditradmin (talk | contribs) |
Cmditradmin (talk | contribs) |
||
| Line 12: | Line 12: | ||
<br clear='all'> | <br clear='all'> | ||
[[Image:Beta-nonadjacent.png|thumb|200px|Non- Adjacent p orbitals do not interact]] | [[Image:Beta-nonadjacent.png|thumb|200px|Non- Adjacent p orbitals do not interact]] | ||
For atoms that are not adjacent to one another Β is taken to be zero. Thus in the Hückel approximation there is no interaction between nonadjacent atoms. | For atoms that are not adjacent to one another Β is taken to be zero. Thus in the Hückel approximation there is no interaction between nonadjacent atoms. Even if you distort the system so that nonadjacent atoms are very close they will not be considered because there is not bonding. | ||
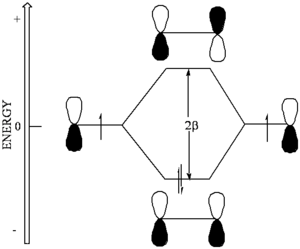
[[Image:Beta-energydiagram.png|thumb|300px|]] | [[Image:Beta-energydiagram.png|thumb|300px|]] | ||
Through this coupling of atomic orbitals, molecular orbitals can formed by a constructive and destructive combination of the atomic orbitals. | Through this coupling of atomic orbitals, molecular orbitals can formed by a constructive and destructive combination of the atomic orbitals. If you begin to fill the orbitals from the lowest to the highest energy you fill the bonding orbital first. This is also known as the highes occupied molecular orbital (HOMO). The antibonding orbital is known as the lowest unoccupied molecular orbital (LUMO). | ||
*The energy of a carbon orbital is taken to be zero, neither stabilized or destabilized. Fluorine would be defined as less than zero. As go to a more electronegative atom you will pull down the energy of the orbital. | |||
*In ethylene the bonding orbital has no nodes and the antibonding orbital has a node that is in between the atoms. | |||
*The bonding orbital is basically stabilized by amount roughly equal to Β and the antibonding orbital is destabilized by a roughly equal amount. In reality, the coupling of the orbitals will be a function orbital overlap which itself depends of how diffuse the orbitals are, their relative orientation and their center to center distance. If bonds are longer the degree of overlap there will be less coupling which decreases the homo / lumo gap. | |||
*An atom more electronegative than carbon will have an p-orbital that lies lower in energy than that of carbon and one more electropositive will have an energy that lies above that of carbon. This will have consequence in the molecular orbitals that are derived from atomic orbitals. | |||
Revision as of 13:02, 19 May 2009
Return to Molecular Orbitals Menu | Next Topic
We will discuss the electronic structure of π-conjugated organic molecules at various levels of complexity. π-conjugated molecules have a sigma electron framework and π electron framework.
Here we start at the simplest level that is basically derived from a Hückel Molecular Orbital approach in order to understand conjugated systems.
In this model, orbitals that are on atoms that are directly σ-bonded to one another and whose p-orbitals are in a plane can interact. This interaction is called the "electronic coupling" between the orbitals and has units of energy.
In the Hückel approximation this energy is the same for all carbon p-orbitals that are adjacent to on another and is called Β.
For atoms that are not adjacent to one another Β is taken to be zero. Thus in the Hückel approximation there is no interaction between nonadjacent atoms. Even if you distort the system so that nonadjacent atoms are very close they will not be considered because there is not bonding.
Through this coupling of atomic orbitals, molecular orbitals can formed by a constructive and destructive combination of the atomic orbitals. If you begin to fill the orbitals from the lowest to the highest energy you fill the bonding orbital first. This is also known as the highes occupied molecular orbital (HOMO). The antibonding orbital is known as the lowest unoccupied molecular orbital (LUMO).
- The energy of a carbon orbital is taken to be zero, neither stabilized or destabilized. Fluorine would be defined as less than zero. As go to a more electronegative atom you will pull down the energy of the orbital.
- In ethylene the bonding orbital has no nodes and the antibonding orbital has a node that is in between the atoms.
- The bonding orbital is basically stabilized by amount roughly equal to Β and the antibonding orbital is destabilized by a roughly equal amount. In reality, the coupling of the orbitals will be a function orbital overlap which itself depends of how diffuse the orbitals are, their relative orientation and their center to center distance. If bonds are longer the degree of overlap there will be less coupling which decreases the homo / lumo gap.
- An atom more electronegative than carbon will have an p-orbital that lies lower in energy than that of carbon and one more electropositive will have an energy that lies above that of carbon. This will have consequence in the molecular orbitals that are derived from atomic orbitals.